Food preservation: a. Principles of food preservation b. Thermal destruction of bacteria - use of low temperature and high temperature. c. Determination of TDP, TDT, D, F, and Z values d. Use of chemicals and antibiotics in food preservation e. Canning
Food preservation:
a. Principles of food preservation
b. Thermal destruction of bacteria - use of low temperature and high
temperature.
c. Determination of TDP, TDT, D, F, and Z values
d. Use of chemicals and antibiotics in food preservation
e. Canning
f. Dehydration
g. Use of radiations
h. Principles of Hazard Analysis and Critical Control Points (HACCP)-
i. Introduction to Tetrapack technology
a. Principles of food preservation:
Foods are mainly composed of biochemical compounds which are derived from plants and animals.
Carbohydrates, proteins and fats are the major constituents of food. In addition, minor constituents such as minerals, vitamins, enzymes, acids, antioxidants, pigments, flavours are present.
Food preservation is the process of treating and handling food to stop or greatly slow down spoilage (loss of quality, edibility or nutritive value) caused or accelerated by micro-organisms.
Preservation usually involves preventing the growth of bacteria, fungi, and other micro-organisms, as well as retarding the oxidation of fats which cause rancidity.
It also includes processes to inhibit natural ageing and discolouration that can occur during food preparation such as the enzymatic browning reaction in apples after they are cut.
Preservative for food may be defined as any chemical compound and/or process, when applied to food, retard alterations caused by the growth of microorganisms or enable the physical properties, chemical composition and nutritive value to remain unaffected
Principles of Food Preservation
The principles of various methods for food preservation are as: Prevention or delay of microbial decomposition
• By keeping out microorganisms (asepsis)
Heating food is an effective way of preserving it because the great majority of harmful pathogens are killed at temperatures close to the boiling point of water.
In this respect, heating foods is a form of food preservation comparable to that of freezing but much superior to it in its effectiveness.
A preliminary step in many other forms of food preservation, especially forms that make use of packaging, is to heat the foods to temperatures sufficiently high to destroy pathogens.
In many cases, foods are actually cooked prior to their being packaged and stored.
In other cases, cooking is neither appropriate nor necessary. The most familiar example of the latter situation is pasteurization.
Conventional methods of pasteurization called for the heating of milk to a temperature between 145 and 149 °F (63 and 65 °C) for a period of about 30 minutes, and then cooling it to room temperature.
In a more recent revision of that process, milk can also be "flash-pasteurized" by raising its temperature to about 160 °F (71 °C) for a minimum of 15 seconds, with equally successful results.
A process known as ultra high pasteurization uses even higher utemperatures of the order of 194 to 266 °F (90 to 130°C) for periods of a second or more.
Application of heat to the foods leads to the destruction of microorganisms.
The specific treatment varies with:
i) The organisms that has to be killed.
ii) The nature of the food to be preserved and
iii) Other means of preservation that may be used in addition to high temperature.
(1) Pasteurization temperature – below 100° C
(2) Heating at about 100°C and
(3) Sterilization temperature abojve 100° C.
a. Pasteurization–below 100°C
Pasteurization is a heat treatment that kills part but not all the microorganisms present and the temperature applied is below 100°C.
The heating may be by means of steam, hot H2O, dry heat or electric currents and the products are cooled promptly after the heat treatments.
The surviving microorganisms are inhibited by low temperature (or) some other preservative method if spoilage is to be prevented.
Preservative methods used to supplement pasteurization include
(i) refrigeration e.g. of milk
(2) keeping out microorganisms usually by packaging the product in a sealed container
(3) maintenance of anaerobic conditions as in evacuated, sealed containers
(4) addition of high concentration of sugar, as in sweetened condensed milk and
(5) presence (or) addition of chemical preservatives e.g. the organic acids on pickles.
Methods of pasteurization
HTST method - High temperature and short time (above 70°C)
LTH method - Low temperature and higher time (or) Holding method (60-70°C)
b. Heating at about 100°C
A temperature of approximately 100°C is obtained by boiling a liquid food, by immersion of the container of food in boiling water or by exposure to flowing steam.
Some very acid foods, e.g., sauerkraut may be preheated to a temperature somewhat below 100° C, packaged hot, and not further heat processed.
Blanching fresh vegetables before freezing or drying involves heating briefly at about 100° C.
c. Sterilization-above 100°C
By this method all microorganisms are completely destroyed due to high temperature.
The time and temperature, necessary for sterilization vary with the type of food.
Temperatures above 100° C can only be obtained by using steam pressure sterilizers such as pressure cookers and autoclaves.
Fruits and tomato products should be noted at 100° C for 30 min.so that the sporeforming bacteria which are sensitive to high acidity may be completely killed.
Vegetables like green peas, okra, beans, etc. being non acidic and containing more starch than sugar, require higher temperature to kill the spore forming organisms.
Continuous heating for 30-90 min. at 116° C is essential for their sterilization.
Before using, empty cans and bottles should also be sterilized for about 30 min. by placing them in boiling water.
Each microorganism present has an optimal temperature for growth and a minimal temperature below which it cannot multiply.
As the temperature drops from this optimal temperature toward the minimal, the rate of growth of the organism decreases and is slowest at the minimal temperature.
Cooler temperatures will prevent growth, but slow metabolic activity may continue.
Most bacteria, yeasts, and molds grow best in the temperature range 16-38oC (except psychrotrophs).
temperatures below 10oC, growth is slow and becomes slower the colder it gets.
The slowing of microbial activity with decreased temperatures is the principal behind refrigeration and freezing preservation.
growth and enzyme reactions are retarded in foods stored at low temperature.
The lower the temperature, the greater the retardation.
Low temperature can be produced by
(a) Cellar storage (about 15° C)
The temperature in cellar (underground rooms) where surplus food is stored in many villages is usually not much below that of the outside air and is seldom lower than 15° C.
It is not enough to prevent the action of many spoilage organisms or of plant enzymes.
Root crops, potatoes, cabbage, apples, onions and similar foods can be stored for limited periods during the winter months.

(b) Refrigerated (or) chilling (0 to 5° C)
Chilling temperature are obtained and maintained by means of ice or mechanical refrigeration.
It may be used as the main preservative method for foods or for temporary preservation until some other preservative process is applied.
Most perishable foods, including eggs, dairy products, meats, sea foods, vegetables and fruits, may be held in chilling storage for a limited time with little change from their original condition.
Enzymatic and microbial changes in the foods are not prevented but are slowed considerably.
 Factors to be considered in co nnection with chilling storage include the temperature of chilling, the relative humidity, air velocity and composition of the atmosphere in the store room, and the possible use of ultra violet rays or other radiations
Factors to be considered in co nnection with chilling storage include the temperature of chilling, the relative humidity, air velocity and composition of the atmosphere in the store room, and the possible use of ultra violet rays or other radiationsThermal death time is the amount of time that is necessary to kill a specific number of microbes at a specific temperature. or Thermal death time is how long it takes to kill a specific bacterium at a specific temperature .
This value is obtained by keeping temperature constant and measuring the time necessary to kill the amount of cells specified.
It was originally developed for food canning and has found applications in cosmetics, producing salmonella-free feeds for animals (e.g. poultry) and pharmaceuticals.
Decimal reduction time (D-value):
The D-value, which denotes the decimal reduction time,
D-value is the time required at a specific temperature and under specified conditions to reduce a microbial population by one decimal.
This is the amount of time that it takes at a certain temperature to kill 90% of the organisms being studied.
The decimal reduction time is dependent on the temperature, the type of microorganism and the composition of the medium containing the microorganism.
F-value
The F value for a process is the number of minutes required to kill a known population of microorganisms in a given food under specified conditions.
This F value is usually set at 12 D values to give a theoretical 12 log cycle reduction of the most heat-resistant species of mesophilic spores in a can of food.
For example, if there were 10,000 spores of a species of spore in a can of food and a 12 D process was given, the initial 10,000 spores (10 4 spores) would be reduced to a theoretical 10-8 living spores per can.
The Z-value.
The Z-value is the increase or decrease in temperature required to reduce or increase the decimal reduction time by one decimal.
It is
a measure of the change in death rate with a change in temperature.
The number of degrees Fahrenheit or Centigrade required for a thermal death time curve to traverse 1 log cycle.
This is the temperature increase required to reduce the thermal death time by a factor of 10.
The z-value gives an indication of the relative impact of different temperatures on a microorganism, with smaller values indicating greater sensitivity to increasing heat.
The z-value is obtained by plotting the logarithms of at least 2 D-values against temperature or by the formula:
Z = (T2-T1)/(logD1-logD2)
Where T = temperature and D = D-value
Use of chemicals in food preservation:
Preservative for food may be defined as any chemical compound and/or process, when applied to food, retard alterations caused by the growth of microorganisms or enable the physical properties, chemical composition and nutritive value to remain unaffected by microbial growth.
Some chemicals have been used traditionally since several decades as direct or indirect inhibitors of microbial growth and are still widely used despite their limitations
The majority of food preservation operations used today also employ some kind of chemical additive to reduce spoilage.
Of the many dozens of chemical additives available, all are designed either to kill or retard the growth of pathogens or to prevent or retard chemical reactions that result in the oxidation of foods.
Some familiar examples of the former class of food additives are sodiumbenzoate and benzoicacid; calcium, sodium propionate, and propionic acid; calcium, potassium, sodium sorbate, and sorbic acid; and sodium and potassium sulfite. Examples of the latter class of additives include calcium, sodium ascorbate, and ascorbicacid(vitaminC);butylatedhydroxyanisole(BHA)andbutylated hydroxytoluene (BHT); lecithin; and sodium and potassium sulfite and sulphur dioxide.
Sl.No. | Name of antibiotic | Active against | Effective pH range | Stability |
1. | Chlortetracycline - | Gram + ve and – ve | 4 to 7 |
|
2. | Oxytetracycline - | -do- | -do- |
|
3. | Penicillin | Gram + ve only. | 5 to 7 |
|
4. | Streptomycin and dihydrostreptomycin | Gram – ve o nly | 7 to 9 |
|
5. |
Polymixin | Gram –ve only | 4 to 8 |
|
e. Canning :
Canning is an important, safe method of food preservation if practiced properly.
The canning process involves placing foods in jars and heating them to a temperature that destroys microorganisms that could be a health hazard or cause the food to spoil.
Canning also inactivates enzymes that could cause the food to spoil.
Air is driven from the jar during heating, and as it cools, a vacuum seal is formed. The vacuum seal prevents air from getting back into the product bringing with it microorganisms to recontaminate the food.
To safely home can foods and prevent food-borne illness, research-based canning methods must be followed.
Botulism is the most commonly associated food-borne illness with home canned foods.
The Centers for Disease Control and Prevention reported that there were 210 outbreaks of botulism from 1996 to 2014, of which 145 were linked to home-canned foods.
Food-borne illnesses related to the consumption of home canned foods are often linked to the person canning the foods not following research-based canning instructions, not using pressure canners for low-acid foods, and ignoring signs of spoilage or lack of knowledge about botulism in home canned foods.
Clostridium botulinum bacteria are the main reason why pressure canning is absolutely necessary for canning low-acid food.
The bacterial cells are killed at boiling temperatures, but they can form spores that survive these temperatures.
The spores grow well in low-acid foods, in the absence of air, such as in canned vegetables and meats.
When the spores begin to grow, they produce the deadly botulinum toxin.
These spores can be destroyed by canning the food at a temperature of 240 °F, or above, for the correct length of time.
This temperature is above the boiling point of water (212 °F) so it can only be reached in a pressure canner.
Only two processing methods are recommended for canning food.
These are the boiling water bath and the steam pressure canner.
All other methods are unsafe and should be avoided by the home canner.
Recommended Methods
1.Boiling Water Bath Method:
The boiling water bath method may be used to process high-acid foods.
These foods can be safely prcessed at 212 degrees F (100 C), the maximum obtainable temperature in a water bath canner at sea level.
Any large metal container may be used as a boiling water bath canner if it is deep enough for the water to be well over the tops of jars and there is space for the water to boil freely.
The canner must have a tight-fi tting cover and a metal rack with dividers to separate and hold jars off the bottom of the canner.
The rack prevents jars from bumping together or tipping over during processing and permits the water to totally surround each jar.
The water in the canner should be hot, but not boiling, before loading with jars of food if the jars have been raw-packed.
The water should be boiling if necessary to adjust the water level.
The jars should be covered by 1 to 2 inches of water for the full processing time so the food in the top of the jar and the jar closure will be thoroughly heated.
The water must boil steadily for the full time recommended for the food being canned. The boiling water bath canner is recommended for canning fruits, tomatoes, foods with added vinegar and fermented foods.
Jams, butters, marmalades, conserves and preserves are also processed in the water bath canner.
2. Steam Pressure Canner Method:
A steam pressure canner is used to process foods under pressure at a temperature of 240 degrees F (116 C).
This is the only reliable mevthod to safely process low-acid foods.
A steam pressure canner is a heavy kettle designed to operate safely at pressures greater than one atmosphere.
It must be equipped with an accurate pressure gauge or weight to register the amount of steam inside the canner.
The lid must lock or seal to prevent the escape of steam.
The canner must have a safety valve and petcock or steam valve that can be opened or closed to permit exhausting (venting) air from the canner.
It must also be equipped with a metal rack and dividers to separate and hold the jars off the bottom of the canner.
The rack prevents jars from bumping together or tipping over during processing and permits the steam to flow uniformly over the jars.
Two types of steam pressure canners are available.
One has a dial pressure gauge, and the other has a weighted gauge to control pressure. Both perform satisfactorily.
The canner operator, however, is cautioned to carefully read the manufacturer’s directions that accompany the canner being used.
The types and brands of canners differ somewhat in details of handling. The steam pressure canner is recommended for canning all foods in the low-acid group.
This group includes all vegetables (except tomatoes), protein foods (meat, poultry, fish), mushrooms, soups, and mixed vegetable recipes containing tomatoes.
Methods to Avoid
1.Open Kettle Method:
Open kettle canning is not a safe method of canning any food. In this method, the food is fi rst cooked in an open kettle and then placed in canning jars and sealed without further processing. Spoilage of foods processed by the open kettle method result from underprocessing, jars and lids that are not thoroughly sterilized or spoilage organisms entering the food while it is placed on the jar.
2.Oven Method:
 Oven canning is not a recommended method for
canning any type of food product. It is both unsafe and
dangerous. It is unsafe even for acid foods because the
temperature of the food never becomes hot enough to
destroy food-spoilage organisms. Since the oven is not
a pressure chamber (such as a pressure canner), the food
inside a canning jar in the oven can be heated no higher
than the boiling point of water (212 degrees F at sea level),
regardless of how high the air temperature is inside the
oven. This is a basic law of physics.
In addition to the danger of inadequate processing,
oven canning can be dangerous. Heating foods in sealed
containers causes a pressure buildup inside the container
due to the expansion of the food and entrapped air. If the metal
band is screwed down too tight to permit the air to escape
from underneath the lid, the pressure buildup will cause
the food container to explode. Should the food container
explode while being handled, it could result in serious
injury
Oven canning is not a recommended method for
canning any type of food product. It is both unsafe and
dangerous. It is unsafe even for acid foods because the
temperature of the food never becomes hot enough to
destroy food-spoilage organisms. Since the oven is not
a pressure chamber (such as a pressure canner), the food
inside a canning jar in the oven can be heated no higher
than the boiling point of water (212 degrees F at sea level),
regardless of how high the air temperature is inside the
oven. This is a basic law of physics.
In addition to the danger of inadequate processing,
oven canning can be dangerous. Heating foods in sealed
containers causes a pressure buildup inside the container
due to the expansion of the food and entrapped air. If the metal
band is screwed down too tight to permit the air to escape
from underneath the lid, the pressure buildup will cause
the food container to explode. Should the food container
explode while being handled, it could result in serious
injury
f. Dehydration :
Dehydration is the extraction of moisture
It inhibits the growth of microorganisms and imparts a long storage life.
This is a modern development of smoking and drying.
Some changes that occur during the process of dehydration are:
· Chemical changes
· Browning and flavor changes
· Denaturation of proteins
Concentration on the surface of the food (case hardening)
Dehydration can be done by drying and salting. Evaporation is quickened with the addition of moderate heat which is sometimes provided by natural sunlight. The ultraviolet rays from the sun serve to kill microbes. Modern methods of dehydration use circulating air that is heated just enough to promote dehydration with-out cooking the food. Food preservation by drying is one of the oldest methods used by human beings. Drying is one of the methods used for dehydration.
Drying is the method of nature resorts to preserve foods.
Natural drying was adopted by early man to dry fruits, fish, and meat by exposing them to the sun.
Sun drying is used in many parts of the world for preserving certain foods, such as fruits and nuts. However, this method can be used only if the climatic conditions are hot with low humidity. In many cases foods are pretreated before drying to make the structure more porous and to facilitate the transfer of moisture, thereby speeding the drying rate. Food porosity increases the chance of quick solubility on reconstitution but is at a disadvantage due to increased bulk and shorter storage stability. Vegetables like beans, peas, potatoes, cauliflower, ladies' finger, garlic, onion, and all leafy vegetables can be sundried.
Shrinkage occurs on the surface first and then proceeds to the inner layers. With quick high-temperature drying of food, the surface becomes dry and rigid long before the center dries out.
Dried food pieces may also contain cracks and pores of various diameters. The shrinking and pore-clogging by the solutes is known as core hardening. It can be minimized by gradual drying with low surface temperature.
Foods that lack good structure and are high in sugar content, give an impression of retaining moisture even after the drying process. Fruits like grapes and figs have high sugar content and lack good structure, hence appearing moist even after dehydration.
Complete prevention of these changes is impossible. They can be minimized by using appropriate technology.
A number of drying methods are available; some are suitable for liquids, others for solid foods, or mixtures containing food pieces. The common drier types used for liquid and solid foods may be categorized as the air-convection drier, drum or roller drier, and vacuum drier.
1.Air-Convection drier – In the air-convection drier, hot air supplies the heat for evaporation. Though there are different types of air-convection driers, they all have an insulated enclosure, a means of circulating air through the enclosure, and a means of heating this air.
If liquid, the food may be sprayed or poured into pans or on belts. Food in the form of a fine spray or mist is introduced into a tower or chamber along with heated air. The small droplets come into contact with the hot air, blast off their moisture, become small particles, and drop to the bottom where they are removed. This method can produce a high-quality product even with heat-sensitive products like milk, eggs, and coffee.
2.Drum or Roller drier – Liquid foods, purees, and mashes are dried by this method. The food to be dried is applied, as a continuous thin layer, on to the surface of a revolving drum or between a pair of drums moving in opposite directions heated by steam. The dried layer of food is scraped by a scraper blade positioned at a point on the drum. Foods that are sticky cannot be scraped when it is hot. Such a sticky food becomes brittle when cooled, which facilitates scraping. For heat resistant food products, drum drying is one of the inexpensive dehydration methods.
3.Vacuum driers – This method is quite expensive but gives good quality foods. It consists of a vacuum chamber that can with stand air pressure and contains shelves to hold food. The shelves are heated. The food gets heated by conduction and radiated heat. Liquid foods dehydrated by vacuum drying have a puffed structure and are easily dissolved in water. There is minimum flavor change and heat damage because the low temperature is used in this method.
Dried foods are very convenient as they are lightweight, take up little storage space and can be stored for long periods as emergency foods.
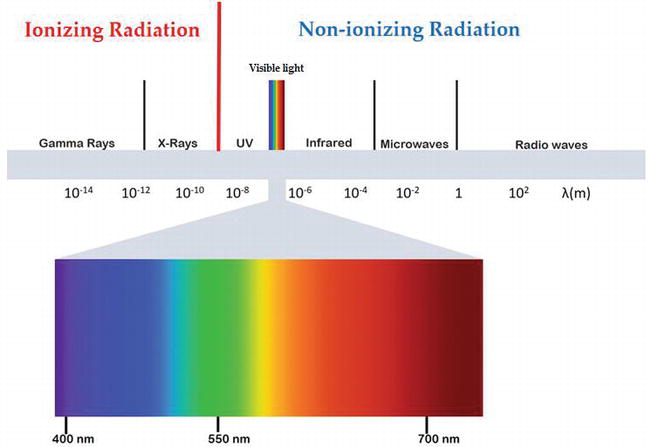
g. Use of radiations:
Many processing methods have been developed to help prevent food spoilage and improve safety.
The traditional methods of preservation, such as drying, smoking, and salting have been supplemented with pasteurization (by heat), canning (commercial sterilization by heat), refrigeration, freezing, and chemical preservatives.
Food irradiation is another technology that can be added to this list
Food irradiation is the process of exposing food to a carefully controlled amount of energy in the form of high-speed particles or electromagnetic radiation.
The choice of the irradiation method will depend on the material needing to be treated.
Thus, to treat the surface or a thin layer of food, one would usually choose beta particles (i.e. electrons). These are easy to produce electronically but they do not have a deep penetrating power.
To treat a bulky product such as an entire sack of spices, one would choose gamma rays or X-rays.
The energy (otherwise known as ionizing radiation) penetrates the food and produces free radicals from the material through which it passes.
Free radicals are highly reactive and very short-lived, so short-lived that they cannot be detected in water-containing food almost immediately after it has been irradiated.
The dose of radiation received is commonly measured in grays.
One gray corresponds to the absorption of one joule of energy in a mass of one kilogram (1Gy = 1J/kg.) The gray has superseded the older unit - the rad (1Gy = 100 rad).
Food Irradiation Applications
The scientific community has defined three levels of food irradiation:

h. Principles of Hazard Analysis and Critical Control Points (HACCP):
Introduction to HACCP
The Hazard Analysis and Critical Control Points (HACCP) system is a logical, scientific approach to controlling hazards in meat production.
HACCP is a preventive system assuring the safe production of food products.
The application of HACCP is based on technical and scientific principles that assure food safety.
An ideal application would include all processes from the farm to the table.
The principle of HACCP can be applied to production, meat slaughter and processing, shipping and distribution, food service, and in-home preparation.
HACCP is a systematic preventative system that uses common sense application of scientific principles.
The most important aspect of HACCP is that it is a preventative system rather than an inspection system of controlling food safety hazards.
Prevention of hazards cannot be accomplished by end-product inspection, so controlling the production process with HACCP offers the best approach.
The application of HACCP is systematic because structured hazard analysis and implementation are provided.
The process is common sense in that each processor understands its operation and is best able to assess controlling the process. HACCP is also science-based and so the controls that are placed in the process should be based on scientific information.
The HACCP system has two major components.
1. The HA of HACCP represents the logic in the hazard analysis which identifies the where and how of hazards.
2. The CCP of HACCP represents the critical control points that provide the control of the process and the proof of the control.
The end objective of HACCP is to make the product as safe as possible and to be able to prove that the product was processed as safe as possible. This does not mean that HACCP provides 100% assurance of food safety to consumers, but does mean that a meat processing company is doing the best job possible for safe food production.
The assurance of safety comes from the process of identifying the hazards, establishing controls for the identified hazards, monitoring the controls, and periodically verifying that the system works.
HACCP focuses on three types of hazards;
1. biological hazards,
2.chemical hazards, and
3.physical hazards.
1. Biological hazards are the type of hazards that receive the most attention in the HACCP system and also present the greatest risk of severity and occurrence. Biological hazards include hazards from pathogens such as bacteria, viruses, yeasts, and molds. Bacteria that receive the greatest attention in the United States include E. coli 0157:H7, Listeria monocytogenes, Salmonella, Staphylococcus aureus, and Campylobacter.
2. Chemical hazards: in meat products could result from the misuse of antibiotics in production, contamination with sanitizers or cleaning agents, or environmental contamination from hydraulic fluids.
Physical hazards: are probably the most recognized by consumers as they usually find this hazard. Glass, metal, and plastic are physical hazards that can occur in meat products.
HACCP was developed by the Pillsbury Company while working on producing foods for NASA for use in space missions in early 1959.
NASA had concerns about food, particularly crumbs, in the space capsule in zero gravity and also food that was free of pathogens and biological toxins that Pillsbury addressed by the use of HACCP.
The concept of HACCP was first presented to the public in the 1971 National Conference on Food Protection.
At that time it was based on three principles.
In 1985, interest in HACCP was renewed when a subcommittee of the Food Protection Committee of NASA issued a report on microbiological criteria.
A National Advisory Committee on Microbiological Criteria for Foods was formed and that committee published a report in 1992 that provided the framework for HACCP as we know it today.
The report by the National Advisory Committee on Microbiological Criteria for Food listed the seven principles of HACCP.
These seven principles become the core of the systematic approach for the application of HACCP.
To start a HACCP system, a company must first write a HACCP plan. Companies may use generic models as resources for developing a plant-specific plan, however, the most useful and successful HACCP plans need to be developed from the very beginning from the plant that will use and implement the plan. To develop a HACCP plan, a team of individuals from within the company, with some assistance from outside experts, conducts five preliminary steps, and applies the seven HACCP principles.
The seven HACCP principles are the most important steps in writing a HACCP plan. The first two steps provide the foundation for the HACCP plan. These two steps are essential since the application of the other HACCP principles depends on the results of the hazard analysis. The remaining five steps are the application steps of the HACCP plan and provide the structure for conducting the workings of the HACCP plan int the processing plant.
Application of the Principles of HACCP
Principle 1 - Conduct a Hazard Analysis
The application of this principle involves listing the steps in the process and identifying where significant hazards are Likely to Occur. The HACCP team will focus on hazards that can be prevented, eliminated, or controlled by the HACCP plan. Justification for including or excluding the hazard is reported and possible control measures are identified.
Principle 2 - Identify the Critical Control Points
A critical control point (CCP) is a point, step, or procedure at which control can be applied and a food safety hazard can be prevented, eliminated, or reduced to acceptable levels. The HACCP team will use a CCP decision tree to help identify the critical control points in the process. A critical control point may control more that one food safety hazard or in some cases more than one CCP is needed to control a single hazard. The number of CCP's needed depends on the processing steps and the control needed to assure food safety.
Principle 3 - Establish Critical Limits
A critical limit (CL) is the maximum and/or minimum value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate, or reduce to an acceptable level the occurrence of a food safety hazard. The critical limit is usually a measure such as time, temperature, water activity (Aw), pH, weight, or some other measure that is based on scientific literature and/or regulatory standards.
Principle 4- Monitor CCP
The HACCP team will describe monitoring procedures for the measurement of the critical limit at each critical control point. Monitoring procedures should describe how the measurement will be taken when the measurement is taken, who is responsible for the measurement and how frequently the measurement is taken during production.
Principle 5 - Establish Corrective Action
Corrective actions are the procedures that are followed when a deviation in a critical limit occurs. The HACCP team will identify the steps that will be taken to prevent potentially hazardous food from entering the food chain and the steps that are needed to correct the process. This usually includes identification of the problems and the steps taken to assure that the problem will not occur again.
Principle 6 - Verification
Those activities, other than monitoring, that determine the validity of the HACCP plan and that the system is operating according to the plan. The HACCP team may identify activities such as auditing of CCP's, record review, prior shipment review, instrument calibration, and product testing as part of the verification activities.
Principle 7 - Recordkeeping
A key component of the HACCP plan is recording information that can be used to prove that the food was produced safely. The records also need to include information about the HACCP plan. The record should include information on the HACCP Team, product description, flow diagrams, the hazard analysis, the CCP's identified, Critical Limits, Monitoring System, Corrective Actions, Recordkeeping Procedures, and Verification Procedures.
i. Introduction to Tetrapack technology:
Tetra Pak is a multinational food packaging and processing sub-company of Tetra Laval, with head offices in Lund, Sweden, and Pully, Switzerland.
The company offers to package, filling machines and processing for dairy, beverages, cheese, ice cream, and prepared food, including distribution tools like accumulators, cap applicators, conveyors, crate packers, film wrappers, line controllers, and straw applicators.
Tetra Pak is currently the largest food packaging company in the world by sales, operating in more than 160 countries.
Dairy products, beverages, ice cream, cheese, food and vegetables, and pet food are examples of products that can be processed or packaged in our processing and packaging lines.
Aseptic packaging can be defined as the filling of a commercially sterile product into a sterile container under aseptic conditions and hermetically sealing the containers so that reinfection is n.
This results in a product, which is shelf-stable at ambient conditions.
The term “aseptic” is derived from the Greek word “septicos” which means the absence of putrefactive micro-organisms.
In practice, generally, there are two specific fields of application of aseptic packaging technology:
1.Packaging of pre-sterilized and sterile products. Examples are milk and dairy products, puddings, desserts, fruit and vegetable juices, soups, sauces, and products with particulates.
2.Packaging of non-sterile products to avoid infection by micro-organisms. Examples of this application include fermented dairy products like yogurt.
Aseptic packaging technology is fundamentally different from that of conventional food processing by canning.
In canning, the process begins with treating the food prior to filling. Initial operations inactivate enzymes so that these will not degrade the product during processing.
The package is cleaned, and the product is introduced into the package, usually hot. Generally, the air that can cause oxidative damage is removed from the interior. The package is hermetically sealed and then subjected to heating.
The package must be able to withstand heat up to about 100°C for high acid products and up to 127°C for low acid products, which must receive added heat to destroy heat-resistant microbial spores.
Packages containing low-acid (above pH 4.5) food must withstand pressure as well.
Advantages of Aseptic Packaging Technology:
The three main advantages of using aseptic packaging technology are:
1.materials, which are unsuitable for in-package sterilization can be used. Therefore, lightweight materials consuming less space offering convenient features and with the low cost such as paper and flexible and semi-rigid plastic materials can be used gainfully.
2. Sterilisation process of high-temperature-short time (HTST) for aseptic packaging is thermally efficient and generally gives rise to products of high quality and nutritive value compared to those processed at lower temperatures for a longer time.
3.Extension of shelf-life of products at normal temperatures by packing them aseptically.
Besides the features mentioned above, additional advantages are that the HTST process utilizes less energy, as part of the process-heat is recovered through the heat exchangers and the aseptic process is a modern continuous flow process needing fewer operators.
Aseptic Processing – Methodology:
Aseptic processing comprises the following:
1.Sterilisation of the products before filling
2. Sterilisation of packaging materials or containers and closures before filling
3 Sterilization of aseptic installations before operation (UHT unit, lines for products, sterile air and gases, filler and relevant machine zones)
4. Maintaining sterility in this total system during operation; sterilization of all media entering the system, like air, gases, sterile water
5.Production of hermetic packages
Sterilization of Products
In aseptic processing, the design to achieve commercial stability is based on the well-founded principles of thermal bacteriology and the integrated effect of time/temperature treatment on spores of micro-organisms.
Pre-sterilization of a product usually consists of heating the product to the desired UHT temperature, maintaining this temperature for a given period in order to achieve the desired degree of sterility, with subsequent cooling, usually to ambient temperature and sometimes to an elevated temperature to achieve the right viscosity for filling.
Heating and cooling should be performed as rapidly as possible to achieve the best quality, depending upon the nature of the product.
A fast heat exchange rate is desired for cost reasons.
Various heat transfer methods are used, but essentially the systems can be divided into direct and indirect heat exchange methods.
Some of the latest methods of sterilization of products include:
• Microwaves
• Electrical resistance heating
• High voltage discharge
• Ultra-high pressure
Sterilization of Aseptic Packaging Materials and Equipment
Sterilisation Agents:
Heat, chemicals, and radiation have been used, alone or in combination, for sterilization of aseptic equipment and packaging materials.
Heat
Initially, the heat was used as the sterilant for aseptic systems as a natural extension of thermal processing.
Product supply lines and fillers are commonly sterilized by ‘moist’ heat in the form of hot water or saturated steam under pressure.
‘Dry’ heat, in the form of superheated steam or hot air, may also be used to sterilize equipment.
However, due to the relatively high dry heat resistance of bacterial endospores, the time-temperature requirements for dry heat sterilization are considerably higher than those for moist heat sterilization.
Since relatively large masses of metal are often present in aseptic filling and packaging systems, high temperatures and relatively long holding periods are necessary to assure that appropriate sterilization has occurred.
Systems employing moist heat are frequently sterilized at temperatures ranging from 121°C to 129°C, while 176°C to 232°C is used for sterilization by dry heat.
In addition, sterilization of air by incineration usually is conducted at temperatures ranging from 260°C to 315°C.
Chemicals
Hydrogen peroxide is the overwhelming choice for use as a chemical sterilant.
Other chemicals that have been used as sterilants, primarily for use in systems for acid food, include various acids, ethanol, ethylene oxide, and peracetic acid.
Hydrogen peroxide is not an efficient sporicide when used at room temperature.
However, the sporicidal activity increases substantially with increasing temperatures.
Therefore, most aseptic packaging systems use hydrogen peroxide (at concentrations of 30 to 35%) as a sterilant for packaging materials followed by hot air (60°C to 125°C) to dissipate residual hydrogen peroxide.
Radiation has been used for decades to decontaminate packaging materials for use in aseptic systems for packing acid and acidified food.
Due to the penetrating powers of gamma-radiation, packages are treated in bulk at commercial irradiators.
A dose of approximately 1.5 Megaradians (Mrad) is commonly used to decontaminate containers for acid and acidified food.
Recently, processes for low acid food aseptic filling and packaging systems are also being accepted.
Doses required to sterilize containers for use with low acid food are considerably higher than those required for acid and acidified food. Other types of radiation are not widely used in aseptic systems. Ultraviolet (UV-C) light has been used to decontaminate food contact surfaces.
Filling :
Once the product has been brought to the sterilization temperature, it flows into a holding tube.
Tube provides the required residence time at the sterilization temperature.
The process is designed to ensure that the fastest moving particle through the holding tube will receive a time/temperature process sufficient for sterilization.
Since there is some loss of temperature as a product passes through the holding tube, the product temperature must be sufficiently high on entering, so that even with some temperature drop, it will still at least be at the prescribed minimum temperature at the exit of the holding tube.
No external heating of the holding tube should take place.
A deaerator is used to remove air, as most products, which are aseptically processed, must be deaerated prior to packaging.
The air is removed to prevent undesirable oxidative reactions, which occur as the product temperature is increased during the process. The deaerator generally consists of a vessel in which the product is exposed to a vacuum on a continuous flow.
Seals and Closures:
Any aseptic system must be capable of closing and/or sealing the package to maintain sterility during handling and distribution.
The integrity of the closure and seal is therefore importance.
The integrity of the heat-seals used in most aseptic
systems is principally influenced by the efficiency of the sealing system used and by contamination of the heat seal area by the product.
To avoid recontamination, the production units, which are tight are required. Two systems are manufactured in the Tetrapak system the longitudinal and the transverse steam.
In the longitudinal system, a flat web of the packaging material is used. This flat material web is formed into a tube, which is sealed longitudinally resulting in a cylinder-shaped structure.
Transversal sealing is done below the level of the product in the packaging material tube. By constantly moving sealing and pressure jaws, the pressure is applied from the outside of the packaging material tube squeezing the product from the sealing area. An electrical impulse is passed through the sealing jaw and heat is transferred from the outside to the inside plastic layer of the packaging material.
Types of Aseptic Packs:
A great variety of packages may be aseptically filled now as listed.
• Carton Boxes:
Some of the existing aseptic carton boxes may now be filled with particulates, also aseptically.
• Bags and Pouches:
Pillow pouches are usually used for packaging of milk; three-sided sealed pouch, however, is suitable also for aseptic packaging of particulates up to particle sizes of 12µ and bag sizes from 1-5 liters. For standing pouches, a Japanese machine uses closed pouches from a reel with sterile interior surfaces, the exterior of which is sterilized in a hydrogen peroxide bath when the web with pouches enters the aseptic cabinet.
The bags are then cut from the web, filled and sealed.
• Cups and Trays:
These are either used pre-made or formed, filled, and sealed in thermoform/ fill/seal machines. Both types of machines exist for filling particulates and also in packs suitable for microwave heating. Usually, polypropylene-based multilayer materials are applied for this purpose.
• Bottles and Jars:
Glass bottles may be aseptically filled with food containing small particles, for instance for baby food.
Jars may be filled with larger particles - 12mm cube size or larger - if one dimension is smaller.
In a recent development, returnable bottles are filled aseptically, which up to now were applied only for UHT – treated milk.
Basically, the same products can be filled into plastic bottles and jars as into glass containers.
Closing is usually done by heat-sealing aluminum lids. For this reason, much attention has to be paid to avoid contamination of heat-sealing rims.
• Metal Cans ;
Aseptic Packaging Materials : materials must meet the following factors :
• The packaging material must be compatible with the product intended to be packed .
• Physical integrity of the package is necessary to assume the containment of the product and maintenance of sterility.
• The packaging material must be able to withstand sterilization and be compatible with the methods of sterilization.
• The package must protect the product from oxygen, also the package must retain the aroma of the product.
Special Need for Plastics in Aseptic Packaging :
However, because plastic material is so important to aseptic packaging, it is useful to discuss some special properties demanded of plastics by the aseptic process itself.
They are as follows:
• Chemical resistance and wetability
• Thermal stability
• Low levels of contaminating microorganisms; and
• Resistance to ionizing radiation.
Market Data on Packaging of Fruit Juices and Milk in Tetra Pak Packages in India :
• White Milk:
The total production of milk in India was 84 billion liters in 2002.
A significant portion (1/3rd) is retained at the farm level itself, and the balance is termed market milk (roughly 55 billion liters).
Of this, only 35 billion liters are liquid white milk, the balance going into milk products, and by-products.
27% of this liquid market milk is packed, the balance is sold loose.
Apart from white milk, the other liquid dairy products like cream, lassi, and buttermilk, flavored milk, etc. are available either in (sterilized) glass bottles, or ordinary plastic pouches, or in Tetra Pak packages.
The pasteurized milk in plastic pouches reaches the consumer mainly through the highly stable and very efficient home delivery channel; a very small proportion is stocked at milk parlors and provision stores to meet the emergency needs of shoppers.
• Juices and Nectars:
The packed juices and nectars market in India is around 33.90 million liters. Of this, 61% or 20.8 million liters are in long-life cartons (predominantly Tetra Pak packages).
The packed juice drinks market in India is around 152 million liters. Popular brands of juice drinks-predominantly mango-are Frooti, Maaza, Slice, and Mangola from the three major players: Parle Agro, Coca Cola, and Pepsico. While juice drinks in glass bottles account for 82.8 million liters, the balance (nearly 43%) is in Tetra Pak packages. The packaged juice drink segment is still only 6% of the total juice drinks market, estimated at 2400 million liters.
Composition of Tetra Pak Aseptic Cartons
Tetra Pak aseptic cartons are made of three basic materials that together result in a very efficient, safe, and light-weight package.
Each material provides a specific function
1. Paper (80%): to provide strength and stiffness
2. Polyethylene (15%): to make packages liquid-tight and to provide a barrier to microorganisms.
3. Aluminium foil (5%): to keep out air, light, and off-flavors
Combining each of these three materials has enabled Tetra Pak to produce packaging material with optimal properties and excellent performance characteristics :
• Higher degree of safety, hygiene and nutrient retention in food •Preserving taste and freshness
• Can be kept for months with no need for refrigeration or preservatives
• Efficient (a filled package weight is 97% product and only 3% packaging material), using a minimum quantity of materials necessary to achieve a given function
• A good example of resource efficiency is its light-weight (among the lightest packages available)
Type of Package Forms available in India :
In India, Tetra Pak offers the following packaging systems currently:
1. TBA: Tetra Brik Aseptic
2. TCA: Tetra Classic Aseptic
3. TFA: Tetra Fino Aseptic
4. TWA: Tetra Wedge Aseptic



Comments
Post a Comment